DHL and Volvo Trucks Join Forces for the Deployment of Heavy E-Trucks
Feb 23, 2021 at 5:30 PMBreakthrough for Digital Control and Safety Technology in Railways?
Feb 24, 2021 at 6:56 PMHow do pharmaceuticals integrate so-called Contract Manufacturing Organizations (CMOs) into the supply chain? What should be considered? What are the approaches? Tim Brandl, a research associate and project staff member at the Institute for Supply Chain Management at the University of St. Gallen (ISCM-HSG), addresses these questions in the following report.
By: Tim Brandl
(St. Gallen) – Collaborating with Contract Manufacturing Organizations (CMOs) allows pharmaceutical companies to flexibly and cost-effectively expand production capacities as needed and gain access to specialized expertise for the development and manufacturing of their products. Due to declining margins in the pharmaceutical industry, CMOs are becoming strategic partners to minimize risks and ensure rapid, scalable product launches. Consequently, CMOs cover an increasingly larger share of the value creation. This symbiosis raises the question for pharmaceutical companies of how to integrate the growing share of outsourced manufacturing into supply chain management and optimize collaboration with CMOs.
Demand-Oriented Control as a Key Element
The performance of pharmaceutical companies is evidently highly dependent on their ability to manage CMOs according to demand. Insufficient and ineffective control and coordination with the service provider can lead to delays and process disruptions. In the worst case, deficiencies in managing CMOs can prevent pharmaceutical companies from meeting their customer commitments due to supply shortages or quality issues. Therefore, a robust approach to managing CMOs is necessary.
It is therefore surprising to find that the systematic management of CMOs has largely been subordinated to industry-specific peculiarities. Since a pharmaceutical company can no longer exert direct influence over the outsourced manufacturing processes, contract manufacturing requires targeted management of the affected supply chains from the outsourcing companies. Unplanned, short-term measures, such as overtime or a subsequent reprioritization of production orders, are not possible due to the independent capacity management of the CMO.
Maturity of Current CMO Management is Limited
A look into practice shows that many pharmaceutical companies are still far from systematically planning and managing the supply chain and CMOs along the product lifecycle. Excellence in CMO management means breaking down the goals of pharmaceutical companies into concrete performance requirements for CMOs. It is essential to underpin supply chain management practices with appropriate metrics, responsibilities, and process patterns. A systematic approach is necessary to determine a suitable set of key figures, measures, and methods. The factors that determine the selection of suitable KPIs and management methods are diverse and include specific product characteristics, outsourcing decision goals, the relationship with CMOs, and other aspects.
Segmentation Simplifies Management by Pharmaceutical Companies
As pharmaceutical companies strive to focus on their core competencies and reduce the operational effort for actively managing their supply chain, the resources they can invest in managing the external supply chain are limited. Therefore, segmenting the CMO supplier base is advisable to cap the effort required to monitor multiple CMOs in the tension between standardization and individualization of the management approach. The segments are typically formed based on factors such as dependency, financial significance, risk, or performance. The use of KPIs and management methods is thus tailored to a specific segment. While supplier segmentation helps categorize CMOs and supports the prioritization of resources for their management, it cannot consider all factors necessary for demand-oriented management of a CMO.
Requirements Vary Along the Product Lifecycle
The main reason for this lies in the dynamics of contract manufacturing relationships. Specific requirements for external production capacities are placed along the product lifecycle. For example, during a market launch, the focus is initially on maintaining stable quality requirements with small quantities and new production methods, as well as responding flexibly to demand developments. In contrast, for generics in a mature product lifecycle phase, the ability to produce large quantities cost-effectively and to adjust to stagnating or declining demand is more important. Consequently, the performance requirements for CMOs change significantly along the pharmaceutical product lifecycle.
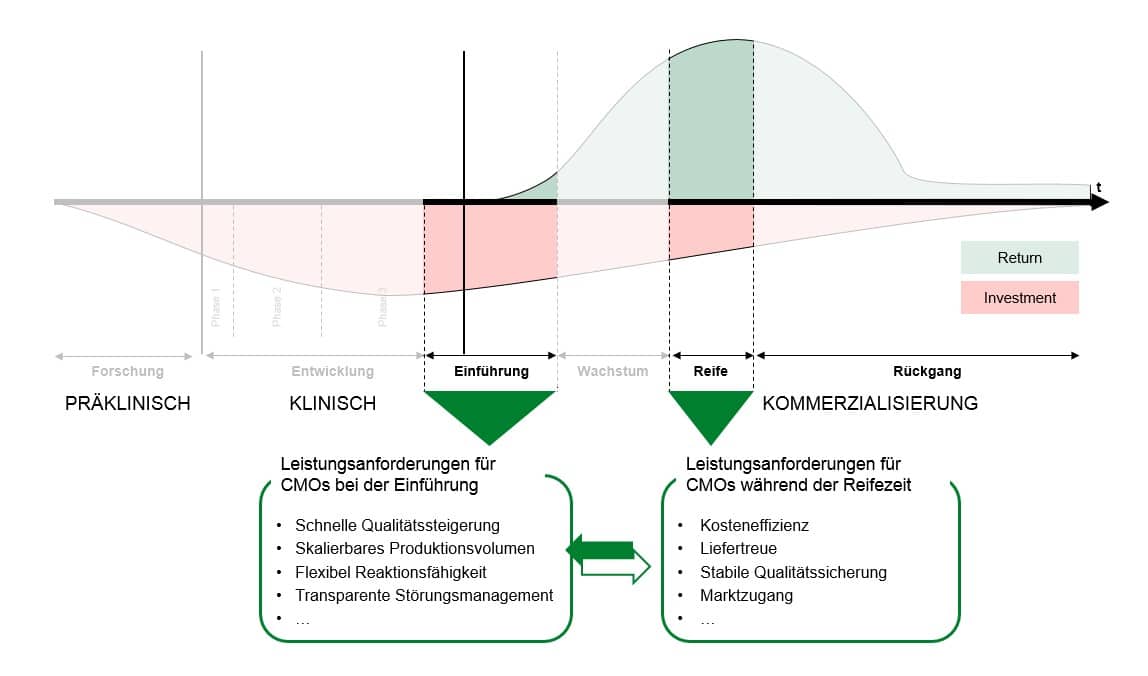
Differentiation of performance requirements for CMOs along the product lifecycle
Studies in the automotive sector have shown that the impact of a static set of KPIs and management methods on supplier performance changes over time due to shifting performance requirements, increasing the risk of disruptions in the supply chain. Since the average pharmaceutical product lifecycle is subject to even greater fluctuations in performance requirements than most components in the automotive sector, the need for dynamic management of CMOs is comparatively high. Therefore, a product lifecycle-based approach to CMO management seems particularly suitable for enabling excellent management of CMOs in pharmaceutical supply chains.
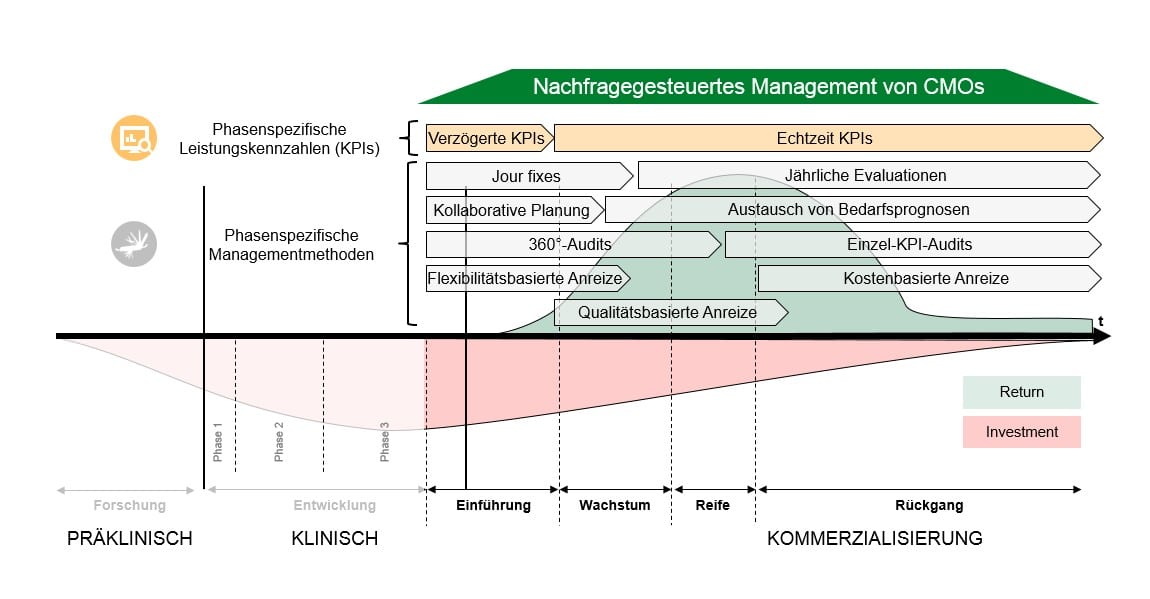
A product lifecycle-based approach for demand-oriented management of CMOs
A product lifecycle-based management of CMOs can be derived from the planning of supplier relationships and considers the most important formal and informal performance requirements for a CMO along the product lifecycle. From this transparent basis, individual sets of management methods emerge that enable efficient control of CMOs and flexibly adapt supply chain management, for example, when a product transitions from patent protection to free competition.
Targeted Control Reduces Risk of Failure
The advantage of product lifecycle-based management of CMOs is that the selection of suitable management methods is directly aligned with the requirements of the product’s manufacturing process, leading to targeted control of CMOs and thus reducing the risk of quality issues and delivery delays. In contrast, widely used segment-based approaches can support resource allocation but do not allow for comparable demand-oriented control of CMOs.
CMOs have established themselves as a cornerstone of the manufacturing of pharmaceutical active ingredients and products. With the increasing complexity and relevance of contract manufacturing in pharmaceutical supply chains, managing CMOs to a standard of excellence becomes necessary. Product lifecycle-based management of CMOs has the potential to establish itself among best practices by placing the requirements of the manufacturing process at the center of management considerations.
Photos/Graphics: © Adobe Stock (cover image) and Institute for Supply Chain Management at the University of St. Gallen (ISCM-HSG)
Tim Brandl is a research associate, project manager, and doctoral candidate at the Institute for Supply Chain Management at the University of St. Gallen. Since 2019, he has been researching operations management and controlling of manufacturing supply chains there. He studied industrial engineering and logistics at the Technical University of Dortmund and was active at the Fraunhofer Institute for Material Flow and Logistics there from 2015 to 2019. https://iscm.unisg.ch







